In high-stakes industries like automotive, aerospace, and precision manufacturing, quality isn’t just a requirement, it’s the backbone of success. Quality Core Tools are more than compliance checkboxes; they’re practical, data-driven methods used throughout the product lifecycle to ensure consistent quality, reduce variation, and prevent defects.
From product design to final inspection, these tools play a crucial role in aligning manufacturing processes with customer expectations and international standards. With increasing complexity in global supply chains and shrinking tolerance for error, mastering these tools has become vital for companies aiming to stay competitive.
In this guide, we’ll break down the most important quality tools starting with the core five and moving into additional techniques used daily by high-performing teams
What Are Quality Core Tools?
Quality Core Tools are a standardized set of quality control methodologies developed primarily by AIAG (Automotive Industry Action Group). Originally created to support the automotive industry, these tools are now widely used across multiple manufacturing sectors.
The 5 traditional core tools include:
- APQP – Advanced Product Quality Planning
- FMEA – Failure Mode and Effects Analysis
- MSA – Measurement System Analysis
- SPC – Statistical Process Control
- PPAP – Production Part Approval Process
They work together across a product’s lifecycle, from concept to delivery. But in practice, quality professionals use more than just five.
List of Top Quality Core Tools
1. APQP – Advanced Product Quality Planning
Advanced Product Quality Planning (APQP) is the cornerstone of proactive quality assurance in the manufacturing world. Originally developed by AIAG for the automotive industry, it’s now widely adopted in aerospace, defense, and complex manufacturing sectors. APQP ensures that quality is designed into the product and process from day one not inspected later.
The APQP framework guides cross-functional teams through structured planning, risk assessment, and validation across a product’s development lifecycle. It’s all about aligning customer expectations with internal capabilities, right from concept to launch. When implemented correctly, APQP reduces surprises during production, improves launch readiness, and builds confidence across the supply chain.
Key Phases of APQP:
- Plan & Define Program – Collect voice of customer (VOC), define goals, and identify key requirements
- Product Design & Development – Develop and review design, conduct Design FMEA, and prototype validation
- Process Design & Development – Create manufacturing process flow, conduct Process FMEA, and define control plans
- Product & Process Validation – Run pilot production, collect Measurement System Analysis (MSA) data, and implement Statistical Process Control (SPC)
- Feedback, Assessment & Corrective Action – Monitor performance, address issues, and apply lessons learned
Why It’s Important:
- Ensures early identification and mitigation of risks
- Strengthens collaboration between engineering, manufacturing, and quality teams
- Prevents costly changes late in the process
- Required by IATF 16949 for new product launches
2. FMEA – Failure Mode and Effects Analysis
Failure Mode and Effects Analysis (FMEA) is a structured, step-by-step technique used to identify and evaluate potential failures in a product or process before they occur. It’s a proactive approach that helps teams anticipate issues, assess the risks associated with each failure mode, and prioritize actions to reduce or eliminate them. Originally introduced in the aerospace and automotive sectors, FMEA has become a standard tool across many quality-driven industries.
There are different types of FMEAs tailored to different stages of development. Design FMEA (DFMEA) targets possible design weaknesses that could result in functional issues or safety problems. Process FMEA (PFMEA) focuses on failures that might arise during manufacturing, assembly, or testing. There’s also System FMEA for analyzing complex, interrelated systems and FMEA-MSR (Monitoring and System Response), which is particularly useful for products that require real-time monitoring, like automotive electronics.
In 2019, AIAG and VDA introduced a harmonized 7-step FMEA approach to streamline practices globally. This structured method enhances collaboration and makes FMEA results more robust and consistent.
The 7-Step FMEA Process Includes:
- Planning and Preparation – Define the scope, boundaries, and objectives. Assemble a multidisciplinary team.
- Structure Analysis – Understand the product or process hierarchy and relationships.
- Function Analysis – Identify and list all intended functions and performance requirements.
- Failure Analysis – Determine potential failure modes, their causes, and effects.
- Risk Evaluation – Rate severity, occurrence, and detection to calculate risk priority numbers (RPNs).
- Optimization – Recommend actions to eliminate or mitigate high-risk failure modes.
- Results Documentation – Record decisions, actions taken, and updated risk evaluations for traceability.
Why It’s Essential:
- Detects and mitigates risk early in development before costly failures occur
- Improves product reliability and ensures customer satisfaction
- Essential for compliance with IATF 16949, ISO 9001, and other global quality standards
- Supports continual improvement and robust design validation
3. PPAP – Production Part Approval Process

The Production Part Approval Process (PPAP) is a standardized method used primarily in automotive and manufacturing industries to confirm that a supplier’s production process consistently delivers parts that meet customer requirements. It ensures both the product and the process are ready for mass production.
PPAP is typically required when introducing a new part, changing design or process elements, or relocating production. It minimizes risks by validating the entire manufacturing process through documented evidence.
Key Elements of PPAP:
- Design Records: Includes ballooned drawings for inspection.
- DFMEA & PFMEA: Failure mode analyses for design and process risks.
- Process Flow Diagram & Control Plan: Visual and control documentation for each step.
- Measurement System Analysis (MSA): Ensures measuring tools are accurate and reliable.
- Dimensional and Test Results: Verifies all part features and performance.
- Initial Process Studies: Confirms stability and capability of key processes.
- Part Submission Warrant (PSW): Final approval form summarizing the entire submission.
4. Statistical Process Control (SPC)
Statistical Process Control (SPC) is a quality tool used to monitor and control manufacturing processes through the application of statistical methods. Rather than relying solely on post-production inspection, SPC helps detect and correct problems during the process itself, preventing defects before they happen.
At its core, SPC uses control charts and process data analysis to distinguish between normal variation and unexpected shifts that may indicate a problem. When applied correctly, SPC leads to more consistent output, reduced waste, and greater efficiency.
Types of Variation:
- Common Cause Variation: Natural fluctuations inherent to the process, usually small and predictable.
- Special Cause Variation: Unexpected changes due to specific issues like equipment failure, human error, or raw material defects. These must be investigated and corrected.
Key Components of SPC:
- Control Charts: Visual tools used to track process performance over time and detect unusual behavior
- Process Capability Analysis: Assesses whether a stable process can consistently meet specifications.
- Detection Rules: Built-in statistical rules help identify when a process is out of control.
Benefits of SPC:
- Early detection of process issues
- Reduction in rework and scrap
- Data-driven decision-making
- Greater process stability and product consistency
SPC can be applied beyond manufacturing as well, including in healthcare, finance, and IT, wherever measurable processes require quality control.
Suggestions to read : Social Media Analytics Tools
5. Measurement System Analysis (MSA)
Measurement System Analysis (MSA) is a fundamental quality tool used to assess the accuracy, precision, and reliability of measurement systems. The purpose of MSA is to ensure that the data collected from inspections or testing is trustworthy enough to support quality-based decisions. If measurements are inconsistent or inaccurate, they can lead to incorrect conclusions, poor product quality, or costly rework.
MSA digs into every component of the measurement process equipment, personnel, environment, and method to uncover potential sources of variation. It’s essential for validating that your measurement systems are fit for use before relying on the data they generate.
What It Analyzes:
- Instruments (calibration status, fixturing, setup)
- Operators (training, interpretation consistency)
- Procedures (test methods and execution)
- Samples (material stability, preparation differences)
- Environment (temperature, humidity, noise, lighting)
- Management (quality systems, oversight, standards)
Common Techniques Used:
- Gage R&R (Repeatability & Reproducibility)
- Calibration studies
- ANOVA-based analysis
- Attribute gage studies
- Destructive testing evaluations
6. Root Cause Analysis (RCA)
Root Cause Analysis (RCA) is a structured problem-solving method used to identify the underlying cause of an issue, rather than just treating its symptoms. The main purpose of RCA is to find out why a problem occurred in order to develop long-term solutions that prevent recurrence. It’s a key quality tool in both manufacturing and service environments, where understanding failures leads to better systems and fewer disruptions.
RCA is typically triggered by significant problems, such as equipment failure, quality defects, safety incidents, or recurring customer complaints. Instead of quick fixes, RCA digs deeper into physical causes (e.g. equipment malfunction), human errors, or organizational weaknesses (e.g. policy gaps) to stop the problem at its source.
Purpose: To identify and eliminate the root causes of problems, not just their symptoms, and implement lasting corrective actions to prevent recurrence.
Key Steps in RCA:
- Clearly define the problem
- Assemble a cross-functional analysis team
- Collect and analyze relevant data
- Identify all possible contributing causes
- Determine the true root cause(s)
- Develop and implement corrective actions
Popular RCA Techniques:
- 5 Whys – Ask “why” repeatedly to drill down to the fundamental cause
- Fishbone Diagram (Ishikawa) – Categorize potential causes visually into logical groups
- Failure Mode and Effects Analysis (FMEA) – Anticipate failures and their impact systematically
- Fault Tree Analysis (FTA) – Use logic diagrams to trace causes backward from the problem
- Causal Factor Tree – Build a structured sequence of contributing events
- Change Analysis – Examine what changed around the time the issue emerged
- Event Analysis – Reconstruct timeline of a specific incident
- Barrier Analysis – Identify missing controls that could have prevented the issue
- DMAIC (Define, Measure, Analyze, Improve, Control) – Use Six Sigma framework for structured problem solving
- Kepner-Tregoe – Structured four-step method to find root cause and evaluate solutions
7. Control Plan
A Control Plan is a structured document that outlines how to monitor and control key process and product characteristics during manufacturing. Its main purpose is to ensure that production remains consistent, predictable, and within specification. It defines what to measure, how often, using which method, and what to do if results fall outside limits. It helps manufacturers prevent defects, maintain quality standards, reduce process variation, and ensure customer requirements are continuously met. Control Plans are essential in high-precision industries like automotive and aerospace where consistency and compliance are critical.
What It Includes:
- Process and product characteristics to control
- Methods of measurement and inspection
- Control techniques (e.g., SPC, audits)
- Sampling frequency and sample size
- Reaction plan for nonconforming result
- Roles and responsibilities
Types of Control Plans:
- Prototype – Created during early product development
- Pre-launch – Used during pilot production
- Production – Used during full-scale production
8. Layered Process Audits (LPA)
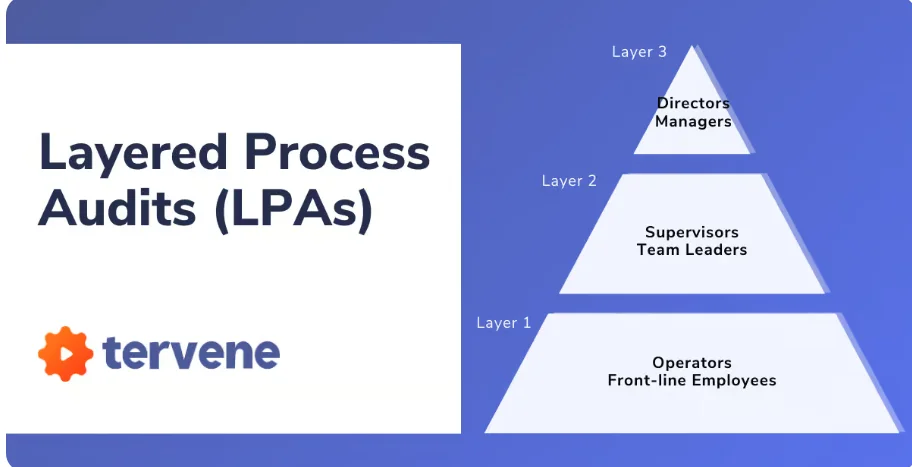
Layered Process Audits (LPA) are a structured auditing approach used to ensure that critical manufacturing processes are being followed exactly as intended — every shift, every day. Unlike traditional quality audits that happen occasionally, LPAs are performed frequently and by different levels of personnel — including line supervisors, quality leads, and senior managers. The main goal is to drive consistency in operations and catch process deviations early before they turn into product defects. By focusing on a small set of critical-to-quality checks, LPAs make auditing quick, actionable, and highly effective.
LPAs are especially important in high-volume or safety-critical manufacturing where even minor deviations can lead to costly recalls or compliance issues. They also promote a shared responsibility for quality across departments, rather than leaving it solely to the quality team.
Purpose:
To catch process deviations early and reinforce standard operating procedures through routine, multi-level audits.
Why It’s Important:
- Ensures processes are followed consistently across shifts and teams
- Reduces variation and defects at the source
- Builds a culture of accountability and ownership for quality
- Provides real-time visibility into operational discipline
9. Process Capability
Process Capability is a statistical measure that evaluates how well a process can produce outputs within specified limits. It reflects the inherent variability of a stable process in relation to customer or engineering specifications. Typically expressed through indices like Cp, Cpk, or Ppk, it helps quantify whether a process consistently meets quality expectations under normal conditions.
What It Analyzes:
- Measures the natural variation in a process output.
- Compare this variation against upper and lower specification limits (USL/LSL).
- Determines if the process is capable of consistently producing within required tolerances.
Key Features:
- Requires the process to be in statistical control (i.e., only common cause variation).
- Uses histograms and control charts to visualize spread and stability.
- Involves collecting sufficient data (typically ≥17 points) under normal conditions.
- Common indices:
- Cp: Potential capability (ideal conditions).
- Cpk: Actual capability, adjusted for process mean shift.
- Ppk: Long-term process performance.
Why It Matters:
Process Capability is critical for understanding how reliable and predictable a process is. It supports quality assurance, helps reduce defects, and ensures compliance with regulatory or customer demands.
10. MasterControl QMS
What It Analyzes:
MasterControl Quality Excellence is an AI-enhanced Quality Management System (QMS) designed specifically for life sciences industries. It streamlines quality processes such as document control, training, risk management, CAPA, audits, and regulatory compliance into one centralized platform. The primary aim is to help companies bring quality products to market faster while ensuring compliance and reducing operational risks.
Key Features:
- AI-Powered Quality Event Management: Customize workflows with no-code tools, automate routing, and track deviations with AI trend analysis.
- Advanced Document Management: Automates version control, real-time collaboration, eSignatures, archiving, and translation.
- Training Management System: Automates training based on document changes, tracks competencies, and supports AI-assisted exam generation.
- Integrated Audit & Risk Management: Enables connected auditing and real-time risk mitigation across the QMS.
- Quality Analytics & Reporting: Offers out-of-the-box dashboards, smart KPI tracking, and AI insights for smarter decision-making.
- Validation Innovation: Delivers faster, risk-based validation of the QMS to reduce implementation and upgrade timelines.
Why It’s Important:
MasterControl is critical for industries like pharma, medtech, and biotech that operate under strict regulatory scrutiny. By unifying quality processes under one digital umbrella, it minimizes manual errors, ensures traceability, improves audit readiness, and speeds up compliance workflows.
Packages Available:
- Basic: Document management only
- Standard: Includes change control and training
- Complete: Adds quality event, audit, risk, supplier, and analytics modules
11. Design Failure Mode and Effects Analysis (DFMEA)
DFMEA is a preventive tool used during the design phase to identify potential failure modes, assess their causes and effects, and prioritize risks before a product reaches production or customers. It’s especially valuable when designing new products, modifying existing designs, or addressing past failures. DFMEA helps engineers anticipate issues early, reducing costly late-stage changes or recalls while enhancing product quality, safety, and customer satisfaction.
What It Analyzes
- Function or item purpose
- Design requirements and specifications
- Possible failure modes
- Effects on system and end-users
- Root causes of failures
- Existing prevention or detection controls
Key Features
- Risk scoring via Severity, Occurrence, Detection
- RPN (Risk Priority Number = S × O × D)
- Action planning to address high-risk areas
- Follow-up and re-ranking post-action
- Continuous updates across the design lifecycle
12. Process Failure Mode and Effects Analysis (PFMEA)
PFMEA is a structured method used to identify and reduce risks in manufacturing or assembly processes by analyzing potential failures and their causes before production begins. It is used when introducing new processes, modifying existing ones, or transferring them to new environments. PFMEA helps ensure robust process design, prevent defects, and improve efficiency by assessing severity, occurrence, and detection of process-related risks.
What It Analyzes
- Each process step and its function
- Potential failure modes (e.g., full, partial, degraded failures)
- Effects on the process, downstream operations, and customers
- Root causes of failure (using 6Ms: Man, Method, Material, Machine, Measurement, Environment)
- Existing preventive and detection controls
Key Features
- Risk Priority Number (RPN): Combines Severity × Occurrence × Detection to prioritize risks
- Failure Mode Classification: Identifies critical or special process characteristics
- Root Cause Focus: Uses structured tools (e.g., fishbone diagram) to pinpoint exact causes
- Control Integration: Links to Control Plan for prevention and detection strategies
- Action-Driven: Recommends improvements and tracks completion and effectiveness
- Continuous Improvement: Updates RPN after actions to confirm risk reduction
13. Eight Disciplines of Problem Solving (8D)
8D is a structured, team-based problem-solving method used when customer complaints, internal failures, or regulatory issues arise. It’s applied to identify the root cause of a problem, implement interim containment, and establish long-term corrective actions to prevent recurrence. It is used when recurring or critical problems need urgent resolution and systemic improvement.
What It Analyzes / Purpose
- Problem symptoms and scope
- Root causes (systemic and direct)
- Escape points (why it wasn’t caught earlier)
- Effectiveness of interim and permanent corrective actions
- Process risks and similar system vulnerabilities
Key Features
- Structured 8-Step Process: Includes team formation, problem description, containment, RCA, corrective actions, validation, and prevention.
- Team-Oriented: Cross-functional collaboration enhances problem understanding and solution quality.
- Interim Containment Action (ICA): Temporary fixes to protect the customer until permanent solutions are verified.
- Root Cause & Escape Point Analysis: Uses tools like 5 Whys, Fishbone, and Is/Is Not to isolate failure causes.
- Permanent Corrective Action (PCA): Solutions are risk-assessed and validated before full implementation.
- Knowledge Retention: Lessons learned, updated procedures, and documented resolutions help avoid recurrence.
Why It Matters
8D improves the reliability and quality of products by systematically eliminating root causes and preventing recurring issues. It also strengthens team problem-solving capabilities and builds a culture of continuous improvement through structured learning and resolution tracking.
Benefits of Using Quality Tools
- Reduces scrap, rework, and production downtime
- Enhances customer satisfaction and compliance
- Strengthens supplier relationships
- Improves first-time-right product delivery
- Supports certifications like ISO 9001 and IATF 16949
Challenges in Implementation
- Requires trained staff and cross-functional collaboration
- May involve resistance to new processes
- Some tools are documentation-heavy and time-consuming
- Integration into daily operations needs strong management support
Trends in Quality Tools – 2025 and Beyond
- AI-Powered FMEA and RCA for predictive risk analysis
- Cloud-based PPAP and APQP tools to streamline global collaboration
- Integration with ERP/QMS platforms for real-time quality data
- Mobile-based LPAs to improve audit frequency and reduce errors
- Visualization dashboards for SPC monitorin
Conclusion
Quality Core Tools aren’t just paperwork, they’re the backbone of world-class manufacturing systems. Whether you’re launching a new product or tightening up your process control, these tools provide the structure, discipline, and insight needed to get it right every time. Businesses can lower costs, increase customer trust in their brand, and decrease defects by combining APQP, FMEA, MSA, SPC, and contemporary digital tools. The year 2025 is ideal for doubling down on quality.
Quality will follow if you let the tools take the lead.